The clay’s chemical capacity allows for the possibility of chemical reactions with other compositions. The clay contains high-grade plastic and colloidal properties, as well as large variations in its physical properties, e.g. transformation into nanocomposites.
The radionics capacity refers to the range of frequencies emitted by the crystalline structure of minerals within the clay, enhanced by photonic diffraction and cation exchange.
A high rate of cation exchange (above 130) has been identified in Brazilian Kimberlite Clay.
This cation exchange capacity, which quantifies the number of exchangeable interlayer tetrahedron / octahedron cations in the chemical process of modification of organic or inorganic ions, acts directly on organophilization by ion exchange of interlayer cations by organic cations, mainly surfactants, and pillarization, as it has swelling properties and ion exchange. Both are essential to this process.
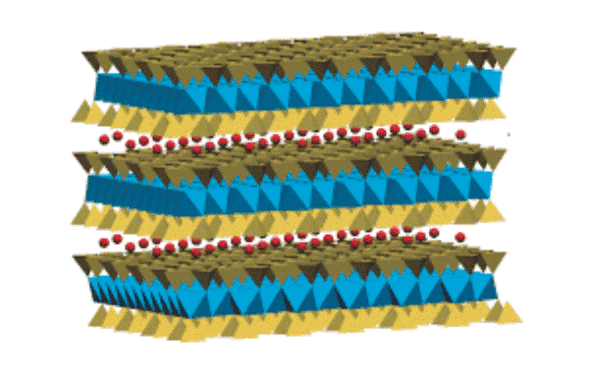
Between the clay’s structural layers are water molecules with oriented and regular arrangements.
They coordinate exchangeable cations and indicate that the water molecules are interacting by hydrogen bonds, which provides organization within the lamellae.
The exchangeable cations control water adsorption in low amounts on the surface of clay. Nanocomposites can be obtained with a high degree of clay dispersion in the matrix and have good mechanical properties at relatively low loading levels because of the affinity between water and clay and through an unprecedented process called dynamic coagulation.